近些年益生菌市场火热,纳豆菌是一种高产蛋白酶的益生菌,能够有效地降解大分子蛋白质,促进营养物质的吸收利用,并能很好地定殖于肠道,改善肠道微生态[5-6]。利用纳豆菌发酵海参开发新型海参精深加工品,将有利于促进海参加工产业升级转型,但发酵过程伴随着复杂的物质转化,发酵前后海参多糖的含量及活性的变化对发酵工艺在海参加工中的应用具有一定的影响。因此,本文采用分光光度法测定发酵海参制备的多糖和海参多糖的羟自由基(·OH)清除能力、1,1-二苯基-2-三硝基苯肼(DPPH)自由基清除能力、2,2-联氮-双-3-乙基苯并噻唑啉-6-磺酸(ABTS)自由基清除能力和铁还原能力,有针对性地对比纳豆菌发酵海参制备的多糖和海参多糖抗氧化活性,为开发新型发酵海参精深加工产品提供理论依据。
1 材料与方法
1.1 材料与仪器
实验所用海参为仿刺参(Apostichopus japonicus),购自大连财神岛集团有限公司;无水葡萄糖、DPPH、维生素C(VC)、乙二胺四乙酸(EDTA)、氯化十六烷基吡啶(CPC)、三水乙酸钠、半胱氨酸、亚铁氰化钾等,购自天津市大茂化学试剂厂;木瓜蛋白酶,购自索莱宝科技有限公司。
千分之一精密电子天平:济宁市裕泽工业科技有限公司;高压蒸汽灭菌锅:山东创美机械科技有限公司;FC3酶标仪:赛默飞世尔科技公司;恒温培养摇床:上海川一实验仪器有限公司。
1.2 实验方法
1.2.1 海参多糖的提取
参照Xiong Q等[7]的方法并稍加改动,进行多糖的提取。在100.00 g海参粉中加入4.38 g EDTA、40.80 g三水乙酸钠、2.36 g半胱氨酸、10.00 g木瓜蛋白酶、3 L蒸馏水,充分混匀后60°C水浴反应24 h。离心后取上清液并加入10%CPC溶液,室温下放置24 h;离心取沉淀,将其溶解于1.5 L 2 mol/L NaCl∶乙醇(VNaCl∶V乙醇=100∶15)溶液中,在混合溶液中加入3倍体积95%的无水乙醇,4°C静置过夜;取下层沉淀,用乙醇洗涤3次,采用6 000~8 000 Da透析袋进行透析,冻干即得海参多糖。
1.2.2 发酵海参制备的多糖的提取
取试管斜面上的纳豆芽孢杆菌,接种至LB液体培养基,35°C培养过夜,作为种子液(菌体浓度约为109 CFU/mL),以4%接种量接种至海参发酵培养基(海参粉3.5 g/L,葡萄糖7.5 g/L,121°C高压蒸汽灭菌后备用),35°C发酵48 h。发酵海参制备的多糖的提取方法同1.2.1海参多糖的提取。
1.2.3 多糖组成成分的测定
1.2.4 DPPH自由基清除率的测定[11]
配制浓度为0.2、0.4、0.6、0.8和1.0 mg/mL多糖溶液与VC溶液,分别吸取2 mL不同浓度发酵海参制备的多糖溶液和海参多糖溶液,加入2 mL避光保存的0.1 mmol/mL DPPH溶液,充分混合均匀,于25°C水浴条件下,避光反应30 min,在517 nm波长下测定吸光值(Optical density,OD),以VC作为阳性对照。
式(1)中:a1为2 mL蒸馏水与2 mL 0.1 mmol/mL DPPH溶液反应的OD值;b1为2 mL样品与2 mL 0.1 mmol/mL DPPH溶液反应的OD值;c1为2 mL样品与2 mL 95%乙醇反应的OD值。
1.2.5 羟自由基清除率的测定[12]
配制浓度为2、4、6、8和10 mg/mL的多糖溶液与VC溶液,分别吸取1 mL不同浓度发酵海参制备的多糖溶液和海参多糖溶液,依次加入3 mL 2 mmol/L FeSO4溶液、3 mL 6 mmol/L水杨酸溶液和3 mL 1 mmol/L H2O2溶液,充分混匀后,于37°C水浴条件下反应30 min,在510 nm波长下测定OD值,以VC作为阳性对照。
式(2)中:a2为以蒸馏水为空白对照的OD值;b2为样品的OD值;c2为以蒸馏水代替H2O2溶液作为本底的OD值。
1.2.6 铁还原力的测定[13]
配制浓度为0.2、0.4、0.6、0.8和1.0 mg/mL的多糖溶液与VC溶液,分别吸取不同浓度发酵海参制备的多糖溶液和海参多糖溶液0.2 mL,加入0.5 mL PBS缓冲液(0.2 mol/L,pH=6.6)和1%亚铁氰化钾溶液0.2 mL,在60°C水浴中处理30 min,水浴后,加入1 mL 10%三氯乙酸溶液并离心。取0.5 mL上清液,加入0.1 mL 0.1%的三氯化铁溶液,再加入0.5 mL蒸馏水,充分混匀后静置10 min,在700 nm波长下测定OD值,以VC作为阳性对照。
式(3)中:a3为样品的OD值;b3为以蒸馏水为空白对照的OD值。
1.2.7 ABTS自由基清除能力测定[14]
配制浓度为0.2、0.4、0.6、0.8和1.0 mg/mL的多糖溶液与VC溶液,分别吸取0.5 mL不同浓度发酵海参制备的多糖溶液和海参多糖溶液,加入0.8 mL ABTS溶液,充分混匀后静置6 min,在700 nm波长下测定OD值,以VC作为阳性对照。
式(4)中:a4为样品的OD值;b4为以无水乙醇为空白对照的OD值。
1.3 数据处理
所有实验均设置3个平行,数据处理采用Excel 2010进行统计学分析,P<0.05为差异显著;画图采用OriginPro 8.5.1软件。
2 结果与分析
2.1 发酵海参制备的多糖和海参多糖的组成成分分析
对发酵海参制备的多糖和海参多糖进行提取,分析发酵海参制备的多糖和海参多糖的组成成分,结果如表1所示。发酵海参制备的多糖和海参多糖蛋白质含量分别为14.87%和13.64%,总糖含量分别为51.91%和49.09%,发酵海参制备的多糖和海参多糖的组成成分无显著性差异(P>0.05)。
表1 发酵海参制备的多糖和海参多糖的成分分析
Tab.1
| 组成成分Composition | 蛋白质/% Protein | 总糖/% Total sugar | 灰分/% Ash |
|---|---|---|---|
| 海参多糖 Polysaccharide from sea cucumber | 14.87±0.23a | 51.91±1.12a | 16.74±0.74a |
| 发酵海参制备的多糖 Polysaccharides from fermented sea cucumber | 13.64±0.41a | 49.09±0.93a | 15.58±0.36a |
注:同一列中相同小写字母表示差异不显著(P>0.05)。
Note:The same lowercase letter in the same column meant no significant difference (P>0.05 ).
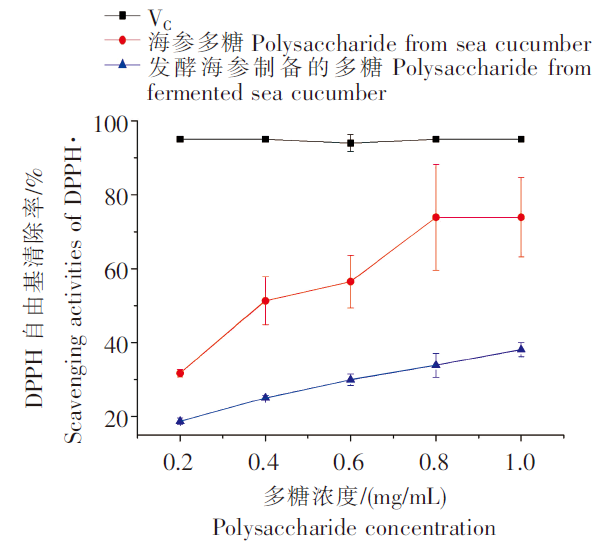
2.2 DPPH自由基清除能力比较
由图1可以看出,VC在较低的浓度下就表现出很强的DPPH自由基清除能力,海参多糖与发酵海参制备的多糖的DPPH自由基清除能力均随多糖浓度的升高而增强,呈明显的量效关系,但是均显著低于VC(P<0.05)。在多糖浓度为1.0 mg/mL时,海参多糖与发酵海参制备的多糖的DPPH自由基清除能力分别为73.92%和38.11%,发酵海参制备的多糖对DPPH自由基的清除能力显著低于海参多糖(P<0.05)。海参多糖的抗氧化活性与其结构息息相关,分子量降低可能降低其抗氧化活性,有研究表明,大分子量的海地瓜粗多糖在同等浓度下表现出最强的DPPH自由基和羟自由基清除能力[4],推测发酵降低了海参多糖的分子量,因而发酵海参制备的多糖的DPPH自由基清除能力降低。
图1
图1
发酵海参制备的多糖和海参多糖的DPPH 自由基清除能力
Fig.1
Scavenging activities of polysaccharide from fermented sea cucumber and sea cucumber on DPPH·
2.3 羟自由基清除能力比较
由图2可以看出,VC在较低的浓度下就表现出很强的羟自由基清除能力,发酵海参制备的多糖和海参多糖的羟自由基清除能力均随多糖浓度的升高而增强,呈明显的量效关系,但是均显著低于VC(P<0.05)。在多糖浓度为1.0 mg/mL时,海参多糖与发酵海参制备的多糖的羟自由基清除能力分别为51.72%和45.94%,发酵海参制备的多糖对羟基自由基的清除能力显著低于海参多糖(P<0.05)。分子量与DPPH自由基和羟自由基清除能力成反比,推测发酵降低了海参多糖的分子量,导致发酵海参制备的多糖的羟自由基清除能力降低。秦裕景等[15]的研究表明,在浓度为4 mg/mL时,VC和从球参中分离出的粗多糖PPP的羟自由基清除率分别为95.14%和30.76%。本文发酵海参制备的多糖和海参多糖羟自由基清除率虽然低于VC,但高于球参的羟自由基清除率。
图2
图2
发酵海参制备的多糖和海参多糖的羟自由基清除能力
Fig.2
Scavenging activities of polysaccharide from fermented sea cucumber and sea cucumber on·OH
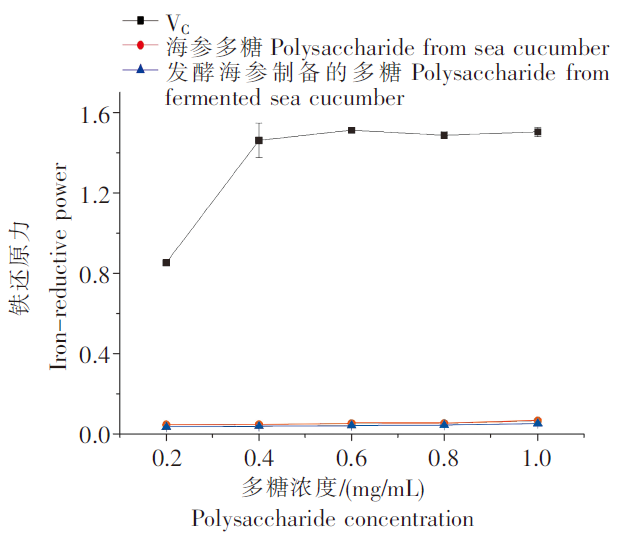
2.4 铁还原力比较
图3
图3
发酵海参制备的多糖和海参多糖的铁还原能力
Fig.3
Icon-reducing power of polysaccharide from fermented sea cucumber and sea cucumber
2.5 ABTS自由基清除能力比较
图4
图4
发酵海参制备的多糖和海参多糖的ABTS 自由基清除能力
Fig.4
Scavenging activities of polysaccharide from fermented sea cucumber and sea cucumber on ABTS+
3 结论
纳豆菌发酵海参制备的多糖和海参多糖均有抗氧化活性,但程度不同,其中发酵海参制备的多糖的羟自由基清除能力和DPPH自由基清除能力低于海参多糖,ABTS自由基清除能力高于海参多糖;两种多糖均没有铁还原能力。发酵海参制备的多糖和海参多糖的含量及组成无明显变化,发酵海参制备的多糖仍具有一定抗氧化活性,说明发酵可以用于海参加工。本研究为新型发酵海参精深加工产品的开发与应用提供了科学依据。
参考文献
Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2
[J].
海参加工产品开发现状及研究进展
[J].海参营养价值和药用价值极高,富含氨基酸、脂肪酸、维生素以及多糖、皂苷、胶原蛋白和脑苷酯等多种活性物质,具有抗氧化、抗肿瘤、抗凝血和调节机体免疫力等多种生理功效。海参加工产品备受消费者青睐,市场前景广阔。文章在阐述海参营养价值和生理功效的基础上,对海参加工产品的开发现状和研究进展进行了系统综述。同时,归纳和总结了海参加工产业发展过程中存在的问题,并对产业未来可能的发展战略进行了展望,以期为海参精深加工产业的发展提供理论参考。
Isolation and optimal fermentation condition of the Bacillus subtilis subsp.natto strain WTC016 for nattokinase production
[J].Nattokinase is a serine protease in the subtilisin family which is produced by Bacillus subtilis subsp. natto and exhibits vigorous fibrinolytic activity that has been suggested to be able to prevent and treat thromboembolic diseases. In this study, WTC016, a spore-forming and rod-shaped bacterium with fibrinolytic activity was successfully isolated from soil, which was identified as Bacillus subtilis subsp. natto based on morphological and physiological tests, and phylogenetic analysis of 16S rRNA and gyrA. According to the growth curve of WTC016, the nattokinase production reached the highest amount in the stationary phase. To optimize the liquid fermentation condition for nattokinase yield of WTC016, further optimal tests of four factors, including the temperature, pH, inoculum size, and loading volume, followed by orthogonal test of all these factors, was performed. The optimal fermentation conditions were determined as 30 °C, 7.0 pH, 2% inoculum size, and 60 mL of loading volume in 250 mL conical flask, which indicates the highest nattokinase production of 3284 ± 58 IU/mL while fermented for 26 h. This work laid the foundation for producing nattokinase using Bacillus subtilis subsp. natto WTC016.
Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto
[J].
Methods of extraction, separation, purification, structural characterization for polysaccharides from aquatic animals and their major pharmacological activities
[J].The further development of fishery resources is a hotspot in the development of the fishery industry. However, how to develop aquatic animal resources deeply is a key point to be solved in the fishery industry. Over the past decades, numerous aquatic animals have gained great attention in the development and utilization of their bioactive molecules which are of therapeutic applications as nutraceuticals and pharmaceuticals. Recent research revealed that aquatic animals are composed of many vital moieties, such as polysaccharides and proteins, which provide health benefits beyond basic nutrition. In particular, aquatic animal polysaccharides are gaining worldwide popularity owing to their high content, ease of extraction, specific structure, few side effects, prominent therapeutic potential and incorporation in functional foods and dietary supplements. Thus, tremendous research on the isolation, identification and bioactivities of polysaccharides has been carried out. This review presents comprehensive viewpoints on extraction, separation, purification, structural characterization and bioactivity of various polysaccharides from aquatic animals, such as sea cucumber, abalone, oyster and mussels. In addition, this review profiled a brief knowledge on both current challenges and future scope in aquatic animal polysaccharides field. The review will be a direction of deep processing in fishery resources, which is a hotspot, but technical bottleneck. Furthermore, the review could be served as a useful reference material for further investigation, production and application of polysaccharides from aquatic animals in functional foods and therapeutic agents.
果胶多糖水热法降解及其产物体外抗氧化性评价
[J].采用水热法降解商品果胶多糖,并对其降解产物的抗氧化活性进行评价。结果表明,水热法降解果胶多糖的最优工艺条件为水热处理温度140 ℃、水热处理时间30 min、pH 6;在此条件下,果胶多糖降解产物得率达46.2%。在此基础上,采用乙醇分级沉淀法对果胶多糖水热处理液进行分离,得到3 种不同分子质量范围的果胶多糖降解产物(S1、S2和S3),其重均分子质量分别为13.4、7.5 kDa和5.7 kDa。以商品果胶多糖和3 种降解产物为研究对象,进行抗氧化性评价,结果表明,S1组分对1,1-二苯基-2-苦基肼自由基的清除率达49.8%,是商品果胶的4 倍;S3组分对超氧阴离子自由基的清除率达58.7%,是商品果胶的10 倍。说明水热降解果胶多糖可显著提高其抗氧化活性,为果渣废弃物的高效利用提供理论依据。
High-value components and bioactives from sea cucumbers for functional foods—a review
[J].Sea cucumbers, belonging to the class Holothuroidea, are marine invertebrates, habitually found in the benthic areas and deep seas across the world. They have high commercial value coupled with increasing global production and trade. Sea cucumbers, informally named as bêche-de-mer, or gamat, have long been used for food and folk medicine in the communities of Asia and Middle East. Nutritionally, sea cucumbers have an impressive profile of valuable nutrients such as Vitamin A, Vitamin B1 (thiamine), Vitamin B2 (riboflavin), Vitamin B3 (niacin), and minerals, especially calcium, magnesium, iron and zinc. A number of unique biological and pharmacological activities including anti-angiogenic, anticancer, anticoagulant, anti-hypertension, anti-inflammatory, antimicrobial, antioxidant, antithrombotic, antitumor and wound healing have been ascribed to various species of sea cucumbers. Therapeutic properties and medicinal benefits of sea cucumbers can be linked to the presence of a wide array of bioactives especially triterpene glycosides (saponins), chondroitin sulfates, glycosaminoglycan (GAGs), sulfated polysaccharides, sterols (glycosides and sulfates), phenolics, cerberosides, lectins, peptides, glycoprotein, glycosphingolipids and essential fatty acids. This review is mainly designed to cover the high-value components and bioactives as well as the multiple biological and therapeutic properties of sea cucumbers with regard to exploring their potential uses for functional foods and nutraceuticals.