1 IgY的分子特性
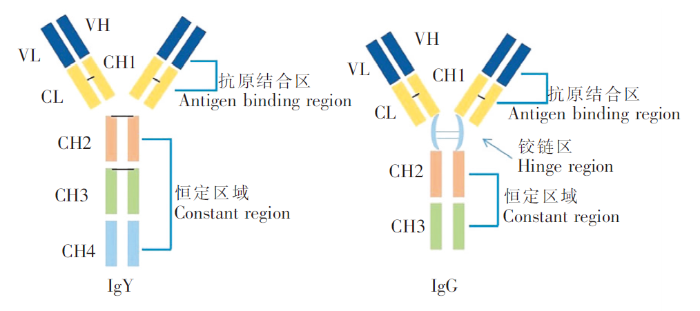
IgY的分子功能与哺乳动物IgG相当,其抗原结合区包含抗原结合位点,恒定区域包含补体活化、调节、致敏或过敏反应所需的柱状细胞结合位点[8]。IgY分子也具有类似于IgG的结构,即两条重链和两条轻链由二硫键连接[9-10]。同时轻链只有一个恒定区(CL)和一个可变区(VL),分子量为18.66 kDa,该结构与IgG相似。IgY和IgG的主要区别在于IgG重链有三个恒定区域(CH1~CH3),而IgY有四个恒定区域(CH1~CH4),这使得IgY(180 kDa)分子量高于IgG(150 kDa)[11]。IgY的抗原结合区与IgG相同,但IgY的CH1和CH2之间的铰链区比IgG柔韧性差。在恒定区域,IgY在CH2和CH3中含有两条糖链,而IgG在CH2中只含有一条糖链(图 1)[12]。由于IgY(CH1~CH4)的主要疏水基团恒定区域大于IgG(CH1~CH3),因此IgY比IgG更疏水,其等电点在5.7~7.6之间[12]。
图1
与使用哺乳动物产生抗体的方法相比,使用鸡作为免疫宿主产生抗体具有更多优势。最显著的优点是IgY是从鸡蛋中获得,同时鸡蛋的收集是非侵入性且具有连续性,对动物无损害行为[6]。同时,蛋鸡的饲养成本低于兔子等哺乳动物,从蛋鸡上获得的抗体总量与牛、羊等大型哺乳动物相当[13]。每只蛋鸡每年可获得17~35 g IgY,其中1%~10%为特异性抗体[14]。连续注射免疫增强剂可使蛋鸡持续10~24个月生产高效价的高免蛋。在功能方面,与哺乳动物IgG不同,IgY不能激活哺乳动物补体系统,与类风湿因子无交叉反应,对蛋白A、蛋白G无亲和力,这为IgY技术在诊断技术等诸多研究领域的应用提供了很大的优势[15-16](表1)。
表1 IgG和IgY的差别
Tab.1
| 参数Parameters | IgG | IgY |
|---|---|---|
| 物种Species | 哺乳动物 | 禽类 |
| 来源Source | 血清 | 蛋黄 |
| 采集方式 Acquisition mode | 对动物有伤害 | 对动物无伤害 |
| 抗体总产量(年)/g Amount of antibody(year) | 5 | 17~35 |
| 特异性抗体总产量/% Amount of specific antibody | <5 | 1~10 |
| 采集持续时间 Acquisition duration | 不连续 | 连续 |
| 分子重量/kDa Molecular weight | 150 | 180 |
| 恒定区Constant region | 3 | 4 |
| 糖链数量 Amount of sugar chain | 1 | 2 |
| 铰链区Hinge region | 有 | 无 |
| 补体激活 Activation of complement | 有 | 无 |
| 类风湿因子激活 Interference with rheumatoid factor | 有 | 无 |
| Protein A/G绑定 Protein A/G binding | 有 | 无 |
| pH | 2.0~11.0 | 4.0~11.0 |
| 热稳定性/℃ Heat stability | 30~80 | 30~70 |
| 疏水性Hydrophobicity | 弱 | 强 |
2 IgY在预防和治疗水生动物疾病中的应用
2.1 IgY在对虾病害防治中的应用
副溶血弧菌是引起虾急性肝胰腺坏死症的病原[20],而IgY能有效抑制细菌的生长和繁殖。Nakamura R等[21]分别用pET32a(+)和pET28b(+)原核表达载体表达副溶血弧菌毒力基因PirA和PirB,经Ni-NTA琼脂糖纯化后,以1 mg·mL-1浓度对蛋鸡进行两轮肌肉注射免疫。收集抗PirA-IgY、抗PirB-IgY和对照组IgY全蛋粉后,将其与饲料按20%或10%浓度混合,用于投喂对虾。预饲养对虾3 d后,以终浓度为1.5×105 CFU·mL-1的副溶血弧菌进行浸泡攻毒。结果表明,第一轮免疫后,投喂抗PirA-IgY、抗PirB-IgY和对照IgY蛋粉的对虾存活率分别为84%、14%和0%;第二轮免疫后,抗PirA-IgY、抗PirB-IgY和对照IgY蛋粉的对虾存活率分别为87%、12%和0%。这些结果证实用抗PirA-IgY和抗PirB-IgY全蛋粉喂养对虾是一种有效的抗副溶血弧菌感染的方法。Hu B C等[22]利用从副溶血弧菌中提取的20 μg·mL-1重组副溶血弧菌外膜蛋白(OMPs)对蛋鸡进行三次肌肉注射免疫,将含有抗OMPs -IgY和对照IgY的蛋粉分别与饲料混合后,饲喂对虾。用浓度为6×107 CFU·mL-1的副溶血性弧菌悬液进行浸泡攻毒。结果表明,饲喂抗OMPs-IgY的对虾肌肉中的细菌载量明显低于对照IgY饲喂组。与此同时,抗OMPs-IgY饲喂组中超氧化物歧化酶活性高于对照IgY饲喂组,这说明抗OMPs-IgY能有效抑制副溶血弧菌的生长。
通过原核表达毒力蛋白和细菌自身结构物质获得的IgY不仅能有效抑制弧菌的感染,使用灭活的全菌也能达到同样的效果。Gao X J等[23]分别将灭活的哈维氏弧菌和副溶血弧菌注射于蛋鸡体内,进行三次免疫后,获得抗哈维氏弧菌IgY和抗副溶血弧菌IgY。再分别以浓度为1×106 CFU·mL-1的哈维氏弧菌和副溶血弧菌进行浸泡攻毒。结果表明,哈维氏弧菌感染48 h后,添加抗哈维氏弧菌IgY处理组蚤状幼体的死亡率为37.3%,未添加的对照组的死亡率为84.0%;副溶血弧菌感染48 h后,添加抗副溶血弧菌IgY处理组蚤状幼体的死亡率为40.0%,未添加的对照组的死亡率为86.7%。Kumaran T等[24]研究证明,灭活哈维氏弧菌免疫蛋鸡获得的IgY也能起到明显的杀菌作用,免疫组感染后30 d累积死亡率为30%,对照组累积死亡率为100%。上述结果表明,用灭活菌免疫蛋鸡后获得的IgY喂养对虾可有效预防哈维氏弧菌和副溶血弧菌感染。Gao X等[25]研究了由阴沟肠杆菌导致的罗氏沼虾发生大规模死亡现象,并探讨了IgY对罗氏沼虾的作用,结果表明IgY效价最高可达1∶213,用其饲喂罗氏沼虾,结果发现阴沟肠杆菌感染96 h后,添加IgY处理组罗氏沼虾蚤状幼体的存活率为67%,明显高于未添加IgY处理组(20%)和阴性对照组(17%),而且肝胰脏的载菌量显著减少。
白斑综合征病毒(White spot syndrome virus, WSSV)是一种在对虾养殖中最具威胁性的传染性病原体,已蔓延至大多数对虾养殖区,造成了巨大的经济损失。目前,为了防止该病原体在虾养殖中的传播,已采用了水消毒和无病原体感染虾幼虫养殖等方法。虽然这些控制措施可以降低WSSV感染的风险,但由于缺乏有效的预防和治疗药物,该病仍在大规模暴发[26]。
缺乏自适应免疫反应系统是造成虾无法抵抗病毒性疾病且大量死亡的主要原因。鉴于这种情况,为虾补充额外的抗体可能是抵御病毒感染的有效方式。抗WSSV特异性IgY已被证明能有效预防WSSV感染。Lu Y A等[27]研究表明,用灭活的WSSV或含有WSSV结构蛋白VP28、VP19和VP15的重组表达免疫原免疫蛋鸡,可刺激蛋鸡产生高滴度中和抗体。将WSSV与上述特异性IgY在室温下孵育1 h后,肌肉注射到对虾体内,结果表明对虾成活率分别为73.3%和33.3%。此外,Lu Y A等[27]研究也证明,当对虾感染WSSV时,通过肌肉注射、口服和浸泡三种不同免疫途径进行抗WSSV特异性IgY被动免疫也会产生较好的保护效果。韦嵩等[28]给凡纳滨对虾喂养添加含有抗WSSV-IgY的饲料,结果显示处理组的免疫相关酶活性均高于对照组,且攻毒感染后的存活率得到显著提高,说明了IgY能够增强对虾的非特异性免疫能力。
2.2 IgY在鱼类病害防治中的应用
嗜水气单胞菌感染引起的细菌性败血症威胁海鲷鱼养殖业的健康发展。Qin Z D等[32]证实使用灭活的嗜水气单胞菌免疫蛋鸡可产生特异性IgY抗体以保护海鲷鱼免受感染,该特异性IgY抗体能有效凝集细菌、显著增强巨噬细胞的吞噬活性、抑制细菌生长甚至杀死细菌;预防免疫实验结果可知经特异性IgY预防的海鲷鱼在感染嗜水气单胞菌后2 d和7 d的存活率分别为80%和60%。Jin L J等[33]研究证明,抗嗜水气单胞菌特异性IgY可以保护鲤鱼免受嗜水气单胞菌感染,与未添加IgY处理组的存活率0%相比,抗嗜水气单胞菌特异性抗体IgY免疫组在感染嗜水气单胞菌后的存活率可达60%。Gan H J等[34]将锦鲤分别浸泡浓度0.2、0.5 g·L-1抗杀鲑气单胞菌特异性IgY后,死亡率分别为50%和70%,而普通蛋黄粉和空白对照组死亡率均高于90%。此外,预防性注射特异性IgY相较于感染后治疗具有更好的抑菌效果。Li X L等[35]研究显示,银鲫感染嗜水气单胞菌前(预防)注射特异性IgY和感染后4 h(治疗)注射特异性IgY的存活率分别为60%和30%,非特异性IgY(n-IgY)和PBS对照组预防和治疗的存活率为0%;银鲫肾脏中的平均细菌数量,在预防中特异性IgY组相较于非特异性IgY和对照组降低了3个数量级,而在治疗嗜水气单胞菌感染(攻毒后)中降低了2个数量级,更好的抑菌性使得特异性IgY在早期细菌预防中发挥的效果相对于治疗有显著性差异。
鳗弧菌是一种革兰氏阴性杆菌,可引起鱼类弧菌病,给鱼类养殖业造成巨大的经济损失。将从病鱼中分离出的鳗弧菌菌株经甲醛灭活后免疫蛋鸡,可获得特异性IgY。Li C H等[39]将鳗弧菌腹腔注射攻毒后,实验鱼经口插管注入不同浓度(100、200 mg·kg-1)的特异性IgY和非特异性IgY。结果表明,特异性IgY治疗组的存活率分别为50%和80%,而非特异性IgY治疗组的存活率仅分别为10%和15%。为了进一步验证特异性IgY的预防保护作用,分别以高浓度的特异性IgY和非特异性IgY连续喂鱼7 d和14 d。预防免疫试验结果表明,特异性IgY处理的鱼的7 d存活率为64%、14 d存活率为5%,这说明抗鳗弧菌特异性IgY在保护动物免受鳗弧菌感染方面起着重要作用。Li C H等[39]研究还证明口服抗鳗弧菌特异性IgY增强了巨噬细胞的吞噬活性,显著减少鱼体内的细菌载量。Gao X等[40]研究结果显示,分别投喂含抗鳗弧菌蛋黄粉、普通蛋黄粉和基础膳食后,半滑舌鳎的死亡率分别为30.0%、86.7%、93.3%。Arasteh N等[41]研究发现,分别在1、3、7、14 d感染鳗弧菌后腹腔注射抗鳗弧菌IgY,虹鳟鱼的死亡率均在30%以下,非特异性抗体和PBS对照组的死亡率均在80%左右。
2.3 IgY在海参病害防治中的应用
用灭活的黄弧菌免疫蛋鸡,获得高效价特异性IgY。Xu L等[46]通过扫描电镜和共聚焦激光扫描显微镜观察,发现特异性IgY可以使细菌团聚并破坏细胞膜,这意味着IgY可以降低细菌活性、杀死细菌。经饲喂质量浓度分别为25、5、1 mg·mL-1的抗黄弧菌IgY后,海参的存活率分别可达77.5%、50.0%和22.5%,而当饲喂质量浓度为25 mg·mL-1的非特异性IgY时,海参的存活率仅为7.5%。饲喂未添加IgY的海参组于12 d内全部死亡,这说明用灭活的黄弧菌免疫蛋鸡可获得高质量浓度的特异性IgY,并对海参弧菌病有预防或治疗作用。
与海参皮肤溃疡综合征相关的另一种有害病原体是灿烂弧菌。Li X Y等[47]研究表明用灭活的灿烂弧菌可获得高质量浓度的特异性IgY。使用灿烂弧菌对海参进行注射攻毒,其中实验组分别注射、浸泡在不同质量浓度的特异性IgY,对照组注射、浸泡高质量浓度的非特异性IgY。结果显示,注射特异性IgY组和浸泡特异性IgY组的海参存活率分别为80%和75%,均高于各自对照组。
2.4 IgY在鲍鱼病害防治中的应用
3 结论与展望
IgY在水生动物疾病细菌和病毒感染防控、治疗中具有广泛的应用,作为抗生素理想的替代品之一具有巨大潜力。然而由于水产养殖过程中,环境因素存在多变性、不确定性和复杂性,IgY在实际生产中的应用还需要重点解决以下问题。
3.1 制备针对多种病原体的多联卵黄抗体
Kotob M H等[50]报道许多水产动物可感染同源病原体或异源病原体,混合感染是水产动物疫病流行的一个重要特点。因此,针对单一病原体,IgY作用效果往往不佳,这就需要根据水生动物常见病原菌制备多种病原体的多联卵黄抗体,降低治疗成本及周期。目前对多联卵黄抗体的作用机制了解还不够深入,未来可以进一步研究其与抗原的结合机制、免疫应答机制等,为优化抗体设计和应用提供理论支持。
3.2 筛选新型佐剂,增强IgY效价及产量
在IgY的工业化生产中,免疫佐剂的选取对增强抗体效价及提高产量起着至关重要的作用。长期以来,弗氏佐剂被用作蛋鸡免疫的标准佐剂,但因价格相对昂贵而不适合规模化蛋鸡免疫。今后通过深入研究免疫机制、探索新型佐剂和优化制备过程,有望为IgY的应用提供更多可能性。
3.3 制定统一的IgY质量标准与检测流程
当前,缺乏统一的IgY质量标准与检测流程,产品质量参差不齐,不利于后续监管。因此,制定统一的IgY质量标准和检测流程是一个系统性的工程,需要多方面的努力和合作。通过不断优化和完善相关标准和流程,可以确保IgY的安全性和有效性,促进其在水产养殖疾病的防治及病害诊断中的应用。
参考文献
Antibiotics in aquatic environments of China: a review and meta-analysis
[J].
Antibiotic use in livestock and residues in food-a public health threat: a review
[J].The usage of antibiotics has been, and remains, a topic of utmost importance; on the one hand, for animal breeders, and on the other hand, for food safety. Although many countries have established strict rules for using antibiotics in animal husbandry for the food industry, their misuse and irregularities in compliance with withdrawal periods are still identified. In addition to animal-origin foods that may cause antibiotic residue problems, more and more non-animal-origin foods with this type of non-compliance are identified. In this context, we aim to summarize the available information regarding the presence of antibiotic residues in food products, obtained in various parts of the world, as well as the impact of consumption of food with antibiotic residues on consumer health. We also aim to present the methods of analysis that are currently used to determine antibiotic residues in food, as well as methods that are characterized by the speed of obtaining results or by the possibility of identifying very small amounts of residues.
Production of anti-SAG1 IgY antibody against Toxoplasma gondii parasites and evaluation of antibody activity by ELISA method
[J].Chicken egg yolk antibody, also known as immunoglobulin Y (IgY), is the predominant class of serum immunoglobulin in birds. IgY has many advantages over mammalian antibodies, such as enhanced immunogenicity conserved mammalian proteins exhibited in birds due to their phylogenetic distance, non-invasive rapid, and economical collection system. However, there are limited studies about IgY production against Toxoplasma, which is a worldwide veterinary and public health problem. In this study, the production of specific IgY antibodies against the surface antigen 1 (SAG1) protein of Toxoplasma gondii and the determination of antibody activity via the enzyme-linked immunosorbent assay (ELISA) method were conducted. According to ELISA, Western blot, and NanoDrop results, specific and higher amounts of IgY antibody against SAG1 were obtained with this study. Considering the advantages of IgY and importance of SAG1 for the diagnosis of toxoplasmosis, it is expected that anti-SAG1 IgY will play an increasing role and gain commercial value in research, diagnostics, and immunotherapy against toxoplasmosis in the future.
Egg yolk antibodies (IgY) and their applications in human and veterinary health: a review
[J].Egg yolk constitutes a relevant alternative source of antibodies. It presents some advantages over mammalian serum immunoglobulins regarding productivity, animal welfare and specificity. The main immunoglobulin present in avian blood (IgY) is transmitted to their offspring and accumulates in egg yolks, which enables the non-invasive harvesting of high amounts of antibodies. Moreover, due to structural differences and phylogenetic distance, IgY is more suitable for diagnostic purposes than mammalian antibodies, since it does not react with certain components of the human immune system and displays greater avidity for mammalian conserved proteins. IgY has been extensively used in health researches, as both therapeutic and diagnostic tool. This article aims to review its applications in both human and veterinary health.Copyright © 2019 Elsevier B.V. All rights reserved.
Ueber natürliche immunität und ihre verwerthung für die immunisirungstherapie
[J].
Production and purification of IgY antibodies from chicken egg yolk
[J].
Chicken immunoglobulin gamma-heavy chains: limited VH gene repertoire, combinatorial diversification by D gene segments and evolution of the heavy chain locus
[J].cDNA clones encoding the variable and constant regions of chicken immunoglobulin (Ig) gamma-chains were obtained from spleen cDNA libraries. Southern blots of kidney DNA show that the variable region sequences of eight cDNA clones reveal the same set of bands corresponding to approximately 30 cross-hybridizing VH genes of one subgroup. Since the VH clones were randomly selected, it is likely that the bulk of chicken H-chains are encoded by a single VH subgroup. Nucleotide sequence determinations of two cDNA clones reveal VH, D, JH and the constant region. The VH segments are closely related to each other (83% homology) as expected for VH or the same subgroup. The JHs are 15 residues long and differ by one amino acid. The Ds differ markedly in sequence (20% homology) and size (10 and 20 residues). These findings strongly indicate multiple (at least two) D genes which by a combinatorial joining mechanism diversify the H-chains, a mechanism which is not operative in the chicken L-chain locus. The most notable among the chicken Igs is the so-called 7S IgG because its H-chain differs in many important aspects from any mammalian IgG. The sequence of the C gamma cDNA reported here resolves this issue. The chicken C gamma is 426 residues long with four CH domains (unlike mammalian C gamma which has three CH domains) and it shows 25% homology to the chicken C mu. The chicken C gamma is most related to the mammalian C epsilon in length, the presence of four CH domains and the distribution of cysteines in the CH1 and CH2 domains. We propose that the unique chicken C gamma is the ancestor of the mammalian C epsilon and C gamma subclasses, and discuss the evolution of the H-chain locus from that of chicken with presumably three genes (mu, gamma, alpha) to the mammalian loci with 8-10 H-chain genes.
Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy
[J].The generation and use of avian antibodies is of increasing interest in a wide variety of applications within the life sciences. Due to their phylogenetic distance, mechanisms of immune diversification and the way in which they deposit IgY immunoglobulin in the egg yolk, chickens provide a number of advantages compared to mammals as hosts for immunization. These advantages include: the one-step purification of antibodies from egg yolk in large amounts facilitates having a virtually continuous supply; the epitope spectrum of avian antibodies potentially grants access to novel specificities; the broad absence of cross-reactivity with mammalian epitopes avoids assay interference and improves the performance of immunological techniques. The polyclonal nature of IgY antibodies has limited their use since avian hybridoma techniques are not well established. Recombinant IgY, however, can be generated from mammalian monoclonal antibodies which makes it possible to further exploit the advantageous properties of the IgY scaffold. Moreover, cloning and selecting the immune repertoire from avian organisms is highly efficient, yielding antigen-specific antibody fragments. The recombinant approach is well suited to circumvent any limitations of polyclonal antibodies. This review presents comprehensive information on the generation, purification, modification and applications of polyclonal and monoclonal IgY antibodies.Copyright © 2012 The International Alliance for Biological Standardization. Published by Elsevier Ltd. All rights reserved.
IgY: clues to the origins of modern antibodies
[J].IgY is the functional equivalent of IgG in birds, reptiles and amphibia, but many aspects of its biology are poorly understood. Recent studies have increased awareness of the genetics and functions of this molecule, and have revealed its position as the ancestor of the uniquely mammalian antibodies IgG and IgE. Here, Greg Warr, Kathy Magor and David Higgins review current knowledge of IgY structure, function and expression in the context of the evolutionary role of this primitive immunoglobulin.
Preparation and mass spectrometric study of egg yolk antibody (IgY) against rabies virus
[J].Rabies virus was used as the antigen to immunize laying chickens. Anti-rabies virus immunoglobulin Y(IgY) was isolated from yolks of the eggs laid by these chickens using a two-step salt precipitation and one-step gel filtration protocol. The purified IgY was reduced with dithiothreitol, and heavy chains (HC) and light chains (LC) were obtained. In addition, the purified IgY was digested with pepsin and the fragment with specific antigen binding properties (Fab) was produced. Using matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOFMS), the average molecular weights of IgY, HC, LC, and Fab were determined as 167 250, 65 105, 18 660, and 45,359 Da, respectively. IgY has two structural differences compared with mammalian IgGs. First, the molecular weight of the heavy chain of IgY is larger than that of its mammalian counterpart, while the molecular weight of the light chain of IgY is smaller. Second, upon pepsin digestion, anti-rabies virus IgY is degraded into Fab, in contrast to mammalian IgG, which has been reported to be degraded into F(ab')(2) under the same conditions.Copyright 2001 John Wiley & Sons, Ltd.
Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine
[J].The production of antibodies (Abs) in chickens and the extraction of specific Abs from egg yolk (IgY Abs) are increasingly attracting the interest of the scientific community, as demonstrated by the significant growth of the IgY literature. This review offers detailed and comprehensive information about IgY-technology, including: a) possibilities for hen keeping in accordance with the Three Rs principles; b) new insights into the IgY transfer mechanism from blood to yolk as a biological basis for the technology; c) the comparative characteristics of IgY Abs and IgG Abs; d) the high efficacy of the technique, in view of the extraordinary amount of IgY Ab produced by one hen in one year (between 20 g and 40 g IgY in total); e) comparisons between the efficacies of IgY Abs and IgG Abs (rabbit, sheep, mouse) in several immunological assays; f) immunisation protocols, as well as the most commonly used IgY-extraction procedures; g) new possibilities for application in human and veterinary medicine, including strategies for the treatment of Helicobacter pylori infection or fatal intestinal diseases in children, particularly in poor countries, for reducing the use of antibiotics, and, in Asia and South America, for producing Abs against snake, spider and scorpion venoms; and h) the use of IgY Abs in various fields of research, also taking into consideration recent developments in South America (particularly Argentina and Cuba) and in Asia.
Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review
[J].Oral administration of chicken egg yolk immunoglobulin (IgY) has attracted considerable attention as a means of controlling infectious diseases of bacterial and viral origin. Oral administration of IgY possesses many advantages compared with mammalian IgG including cost-effectiveness, convenience and high yield. This review presents an overview of the potential to use IgY immunotherapy for the prevention and treatment of terrestrial and aquatic animal diseases and speculates on the future of IgY technology. Included are a review of the potential application of IgY for the treatment of livestock diseases such as mastitis and diarrhea, poultry diseases such as Salmonella, Campylobacteriosis, infectious bursal disease and Newcastle disease, as well as aquatic diseases like shrimp white spot syndrome virus, Yersina ruckeri and Edwardsiella tarda. Some potential obstacles to the adoption of IgY technology are also discussed.Copyright © 2011 Elsevier Inc. All rights reserved.
Peroral immunotheraphy with yolk antibodies for the prevention and treatment of enteric infections
[J].Oral administration of specific antibodies is an attractive approach to establish protective immunity against gastrointestinal pathogens in humans and animals. The increasing number of antibiotic-resistant bacteria emphasize the need to find alternatives to antibiotics. Immunotherapy can also be used against pathogens that are difficult to treat with traditional antibiotics. Laying hens are very good producers of specific antibodies. After immunization, the specific antibodies are transported to the egg yolk from which the antibodies then can be purified. A laying hen produces more than 20 g of yolk antibodies (IgY) per year. These antibodies also have biochemical properties that make them attractive for peroral immunotherapy: They neither activate mammalian complement nor interact with mammalian Fc receptors that could mediate inflammatory response in the gastrointestinal tract. Eggs are also normal dietary components and thus there is practically no risk of toxic side effects of IgY. Yolk antibodies have been shown in several studies to prevent bacterial and viral infections.
Adjuvant effects of various lipopeptides and interferon-γ on the humoral immune response of chickens
[J].The adjuvant effects of various lipopeptides and recombinant chicken interferon gamma (IFN-gamma) on the humoral immune response of laying hens was investigated in four immunization studies. We used the lipopeptide Pam3Cys-Ser-(Lys)4 (PCSL), the conjugate P-Th1 consisting of the lipopeptide P3CS and the T-helper epitope Th1 (FISEAIIHVLHSRHPG), and the conjugate P-Th2 of the lipopeptide P3CSS and the T-helper epitope Th2, which corresponds to the peptide EWEFVNTPPLV, as adjuvants. Human serum albumin (HSA), recombinant bovine somatotropin (RBST), and human immunoglobulin G (IgG) served as antigens in the different experiments. All tested adjuvants enhanced the humoral immune response with various intensities. Chickens showed high antibody titers after the immunization with HSA even without adjuvant, but the adjuvant effects of PCSL and the combination of PCSL and recombinant chicken interferon-gamma (IFN-gamma) were much more pronounced using the antigens RBST and IgG. Especially after the third immunization, higher titers of antibodies were induced by the coadministration of P-Th1 and, to a greater extent, by the combination of PCSL and P-Th1 compared with the use of PCSL. Also, chickens that had received PCSL and P-Th2 showed the highest immune response, even after the second booster. The average concentrations of chicken immunoglobulin Y were significantly higher in 5-mo-old chickens (9.4 mg/mL serum and 10.1 mg/mL egg yolk) compared with 9-mo-old chickens (5.9 mg/mL serum and 5.1 mg/mL egg yolk). The specific serum antibody response was higher in the older chickens than in the younger chickens. Because chicken antibodies are likely to be used increasingly for diagnostic and therapy in the future, lipopeptides and recombinant chicken IFN-gamma may find many applications as adjuvants, thus contributing to the welfare of experimental animals.
Anti-Vibrio and immune-enhancing activity of medicinal plants in shrimp: a comprehensive review
[J].
Current and future perspectives for controlling Vibrio biofilms in the seafood industry: a comprehensive review
[J].
Vibrio and major commercially important vibriosis diseases in decapod crustaceans
[J].
Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques
[J].Vibrio parahaemolyticus is a Gram-negative halophilic bacterium that is found in estuarine, marine and coastal environments. V. parahaemolyticus is the leading causal agent of human acute gastroenteritis following the consumption of raw, undercooked, or mishandled marine products. In rare cases, V parahaemolyticus causes wound infection, ear infection or septicaemia in individuals with pre-existing medical conditions. V parahaemolyticus has two hemolysins virulence factors that are thermostable direct hemolysin (tdh)-a poreforming protein that contributes to the invasiveness of the bacterium in humans, and TDH related hemolysin (trh), which plays a similar role as tdh in the disease pathogenesis. In addition, the bacterium is also encodes for adhesions and type III secretion systems (T3SS1 and T3SS2) to ensure its survival in the environment. This review aims at discussing the V parahaemolyticus growth and characteristics, pathogenesis, prevalence and advances in molecular identification techniques.
Anti-PirA-like toxin immunoglobulin (IgY) in feeds passively immunizes shrimp against acute hepatopancreatic necrosis disease
[J].Acute hepatopancreatic necrosis disease (AHPND), caused by a toxin-producing Vibrio parahaemolyticus strain, has become a serious threat to shrimp aquaculture. The need to regulate antibiotic use prompted the development of alternative ways to treat infections in aquaculture including the use of chicken egg yolk immunoglobulin (IgY) for passive immunization. This study evaluated the protective effect of IgY against AHPND infection in Litopenaeus vannamei (Boone). IgY was isolated from eggs laid by hens immunized with recombinant PirA-like (rPirA) and PirB-like (rPirB) toxins. Whole-egg powders having IgY specific to rPirA (anti-PirA-IgY) and rPirB (anti-PirB-IgY) and IgY from non-immunized hen (control-IgY) were mixed with basal diets at 20% concentrations and used to prefeed shrimp 3 days before the bacterial challenge test. Survival rates of the challenged shrimp fed the anti-PirA-IgY, anti-PirB-IgY and control-IgY diets were 86%, 14% and 0%, respectively. Only the feed containing anti-PirA-IgY protected shrimp against AHPND. Increasing the concentration of rPirA antigen to immunize hens and lowering the amount of egg powder in feeds to 10% consistently showed higher survival rates in shrimp fed with anti-PirA-IgY (87%) compared with the control (12%). These results confirm that addition of anti-PirA-IgY in feeds could be an effective prophylactic method against AHPND infection in shrimp.© 2019 John Wiley & Sons Ltd.
The preparation and antibacterial effect of egg yolk immunoglobulin (IgY) against the outer membrane proteins of Vibrio parahaemolyticus
[J].
Passive immune-protection of Litopenaeus vannamei against Vibrio harveyi and Vibrio parahaemolyticus infections with anti-Vibrio egg yolk (IgY)-encapsulated feed
[J].
Physicochemical properties of anti Vibrio harveyi egg yolk antibody (IgY) and its immunological influence in Indian white shrimp Fenneropenaeus indicus
[J].
Enterobacter cloacae associated with mass mortality in zoea of giant freshwater prawns Macrobrachium rosenbergii and control with specific chicken egg yolk immunoglobulins (IgY)
[J].
A review on the morphology, molecular characterization, morphogenesis and pathogenesis of white spot syndrome virus
[J].Since it first appeared in 1992, white spot syndrome virus (WSSV) has become the most threatening infectious agent in shrimp aquaculture. Within a decade, this pathogen has spread to all the main shrimp farming areas and has caused enormous economic losses amounting to more than seven billion US dollars. At present, biosecurity methods used to exclude pathogens in shrimp farms include disinfecting ponds and water, preventing the entrance of animals that may carry infectious agents and stocking ponds with specific pathogen-free post-larvae. The combination of these practices increases biosecurity in shrimp farming facilities and may contribute to reduce the risk of a WSSV outbreak. Although several control methods have shown some efficacy against WSSV under experimental conditions, no therapeutic products or strategies are available to effectively control WSSV in the field. Furthermore, differences in virulence and clinical outcome of WSSV infections have been reported. The sequencing and characterization of different strains of WSSV has begun to determine aspects of its biology, virulence and pathogenesis. Knowledge on these aspects is critical for developing effective control methods. The aim of this review is to present an update of the knowledge generated so far on different aspects of WSSV organization, morphogenesis, pathology and pathogenesis.
Passive immunization of crayfish (Procambius clarkiaii) with chicken egg yolk immunoglobulin (IgY) against white spot syndrome virus (WSSV)
[J].White spot syndrome virus (WSSV) is a major cause of mortality in shrimp lacking a true adaptive immune response. In this study, high activity egg yolk immunoglobulin (IgY) against WSSV for passive immunization of crustaceans was already prepared as crude and purified product, while an indirect enzyme-linked immunosorbent assay test was used for quality control of IgY activity. The effectiveness of IgY of intramuscular injection, oral administration, and immersion was investigated in crayfish (Procambius clarkiaii) against WSSV. The result showed that the groups treated with IgY from inactivated WSSV and DNA vaccine were, respectively, 20% and 80% mortality, which were significant difference in survival rates (P < 0.05) from the positive control groups. The groups in diet added 10% egg yolk powder and 1% IgY power showed 53.3% and 67.7% mortality, respectively, and the immersion showed 46.7% mortality, which have significantly different compared to the positive groups (P < 0.05). These results indicated passive immunization of specific IgY antibodies through intramuscular injection, oral administration, and immersion have effective to protect crayfish against WSSV. It is noteworthy that IgY as feed additive and immersion solution is useful and feasible methods in practical work. Thus, our results suggest that the passive immunization of crayfish with IgY against WSSV will have potential development to prevent and control WSSV in practical culture.
Aeromonas hemolytic uremic syndrome: a case and a review of the literature
[J].Although rarely, hemolytic uremic syndrome can be induced by Aeromonas. We report a case in a 40-year-old Spanish female produced by Aeromonas veronii bv. sobria and review the previous cases described in the literature. This is the 2nd case described in adults.
Necrotizing fasciitis and sepsis caused by Aeromonas hydrophila after crush injury of the lower extremity
[J].Aeromonas hydrophila is a motile gram-negative bacillus found in water sources that typically causes minor skin infections or gastroenteritis in humans. There are sporadic reports of cases of sepsis or necrotizing fasciitis caused by A. hydrophila but no other cases of severe infection secondary to trauma. The mortality rate of septic shock caused by A. hydrophila approaches 100%.Case report and review of pertinent literature.A patient recently seen at our institution illustrates the features of necrotizing fasciitis and sepsis caused by A. hydrophila after an open femur fracture. We describe the aggressive multi-modality treatment necessary to maximize the likelihood of survival.
The genus Aeromonas: taxonomy, pathogenicity, and infection
[J].Over the past decade, the genusAeromonashas undergone a number of significant changes of practical importance to clinical microbiologists and scientists alike. In parallel with the molecular revolution in microbiology, several new species have been identified on a phylogenetic basis, and the genome of the type species,A. hydrophilaATCC 7966, has been sequenced. In addition to established disease associations,Aeromonashas been shown to be a significant cause of infections associated with natural disasters (hurricanes, tsunamis, and earthquakes) and has been linked to emerging or new illnesses, including near-drowning events, prostatitis, and hemolytic-uremic syndrome. Despite these achievements, issues still remain regarding the role thatAeromonasplays in bacterial gastroenteritis, the extent to which species identification should be attempted in the clinical laboratory, and laboratory reporting of test results from contaminated body sites containing aeromonads. This article provides an extensive review of these topics, in addition to others, such as taxonomic issues, microbial pathogenicity, and antimicrobial resistance markers.
Protective effects of chicken egg yolk immunoglobulins (IgY) against experimental Aeromonas hydrophila infection in blunt snout bream (Megalobrama amblycephala)
[J].
Protection of crucian carp (Carassius auratus Gibelio) against septicaemia caused by Aeromonas hydrophila using specific egg yolk immunoglobulins
[J].
Ulcer disease prophylaxis in koi carp by bath immersion with chicken egg yolk containing anti-Aeromonas salmonicida IgY
[J].
Protection of Carassius auratus Gibelio against infection by Aeromonas hydrophila using specific immunoglobulins from hen egg yolk
[J].
Identification and expression analysis of fetuin B (FETUB) in turbot (Scophthalmus maximus L.) mucosal barriers following bacterial challenge
[J].
Synergistic effect of a combined live Vibrio anguillarum and Edwardsiella piscicida vaccine in turbot
[J].
Oral administration of microencapsulated egg yolk immunoglobulin (IgY) in turbot (Scophthalmus maximus) to combat against Edwardsiella tarda 2CDM001 infections
[J].
Passive protective effect of chicken egg yolk immunoglobulins against experimental Vibrio anguillarum infection in ayu (Plecoglossus altivelis)
[J].
Passive protection effect of anti-Vibrio anguillarum IgY-encapsulated feed on halfsmooth tongue sole(Cynoglossus semilaevi) against V.anguillarum
[J].
Passive immunization of rainbow trout (Oncorhynchus mykiss) with chicken egg yolk immunoglobulins (IgY)
[J].
Betanodavirus: mitochondrial disruption and necrotic cell death
[J].
Features of chicken egg yolk immunoglobulin (IgY) against the infection of red-spotted grouper nervous necrosis virus
[J].
Use of phages to control Vibrio splendidus infection in the juvenile sea cucumber Apostichopus japonicus
[J].
Phenotypic and genetic characterization of bacteria isolated from diseased cultured sea cucumber Apostichopus japonicus in northeastern China
[J].
Immunomodulatory effects of chicken egg yolk antibodies (IgY) against experimental Shewanella marisflavi AP629 infections in sea cucumbers (Apostichopus japonicus)
[J].
Protective effects of chicken egg yolk antibody (IgY) against experimental Vibrio splendidus infection in the sea cucumber (Apostichopus japonicus)
[J].
Health and survival of red abalone, Haliotis rufescens, under varying temperature, food supply, and exposure to the agent of withering syndrome
[J].
Passive immune-protection of small abalone against Vibrio alginolyticus infection by anti-Vibrio IgY-encapsulated feed
[J].
The impact of tetracapsuloides bryosalmonae and Myxobolus cerebralis coinfections on pathology in rainbow trout
[J].